
|
|
|
|
|
|
|
|
|
The energy that drives biological processes is the
foundation of living systems. Every
chemical process that occurs in or around an organism has an impact on how the
cell allocates its metabolic resources.
This principle forms the conceptual basis for regulatory proteins;
non-homeostatic concentrations of a metabolite will induce an appropriate
cellular response through allosteric communication between the effector and DNA
binding sites. Fundamental
thermodynamic information about processes such as these will enable more
accurate predictions regarding the response elicited by a cell to specific
stimuli. This entails a profound
understanding of the general communication pathways that biological systems
utilize. The research in the
Grossoehme lab
aims to develop and implement robust experimental methodologies capable of
determining a complete thermodynamic description of these energies.
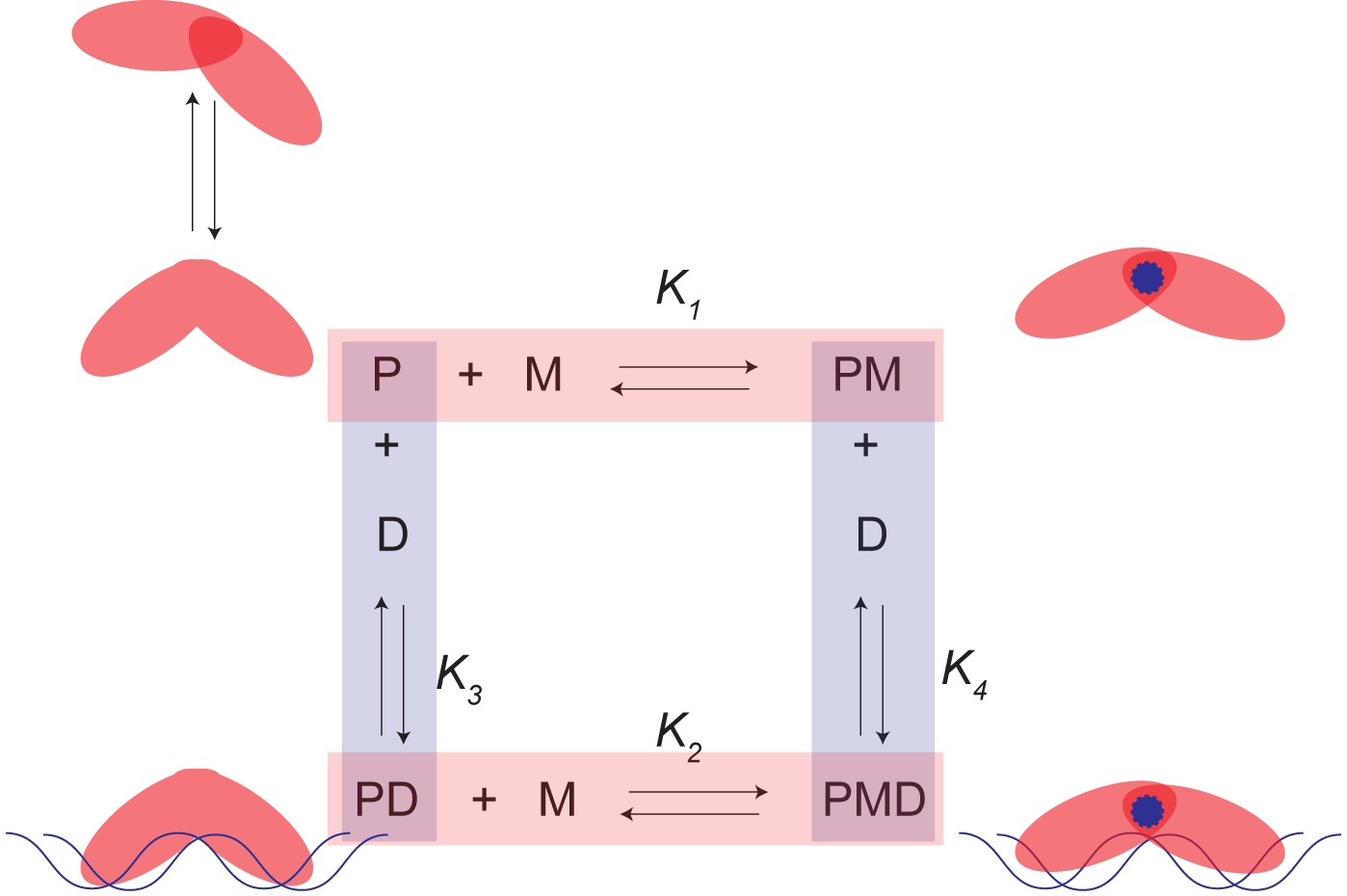
Our approach, shown schematically in the figure just below, assigns each
functional state of an allosteric regulatory protein to a corner of a closed
thermodynamic cycle where P is the apoprotein, PM and PD are the protein-metal
complex and protein-DNA complex, respectively, and PMD is the ternary complex
that may form between the protein, metal and DNA.
In this scheme, each side of the ‘box’ represents a chemical reaction
that can occur in the cell. This
allows us to relate the underlying thermodynamics of one side of the box to
another and ultimately learn something about the energetics that allow the
protein to carry out its function.
Cellular Copper Regulation
Mechanisms of copper homeostasis are of particular
interest due to the need for organisms to devote significant cellular resources
to regulate the concentration of this metal, which stems from the role copper
plays in both essential and toxic processes.
A large amount of information has been obtained about the biochemistry of
copper. Similar to iron, copper’s
usefulness stems from a redox couple within the physiological range of reduction
potentials (ε0 =0.153V), enabling electron transport through
proteinacious copper centers.
However, like iron, this redox couple also creates the possibility for
deleterious effects such as the catalytic production of reactive oxidation
species or inactivation of critical cellular enzymes through interactions with
iron-sulfur clusters. It is therefore critical for cytosolic concentrations to
be tightly regulated by the cell.
Over the last decade, it has been repeatedly demonstrated that Cu+ is
the physiologically relevant oxidation state for membrane transport,
metallochaperone function and gene regulation.
This complicates in vitro
experiments, which have mainly focused on the Cu2+ oxidation state
based on relative stabilities under typical laboratory conditions.
Particularly,
the presence of molecular oxygen will
result in the spontaneous oxidation of Cu+ in a very favorable
process (∆G˚ = -99 kcal mol-1), necessitating strict anaerobic
conditions. Further, Cu+
will participate in a disproportion process (Equation 1).
![]()
Metal Regulation in Streptomyces
coelicolor
Additionally, we are investigating metal regulatory networks in
Streptomyces coelicolor, an organism of interest due to the
observation that
metal
homeostasis
is directly linked to the biosynthesis of coelibactin, the product of a
dedicated polyketide biosynthetic mechanism and precursor to multiple
antibiotics. Notably, the
Actinomyces genus, of which S.
coelicolor is the most widely studied and understood, accounts for roughly
half of the microbial antibiotics discovered to date. This project has two
immediate goals:
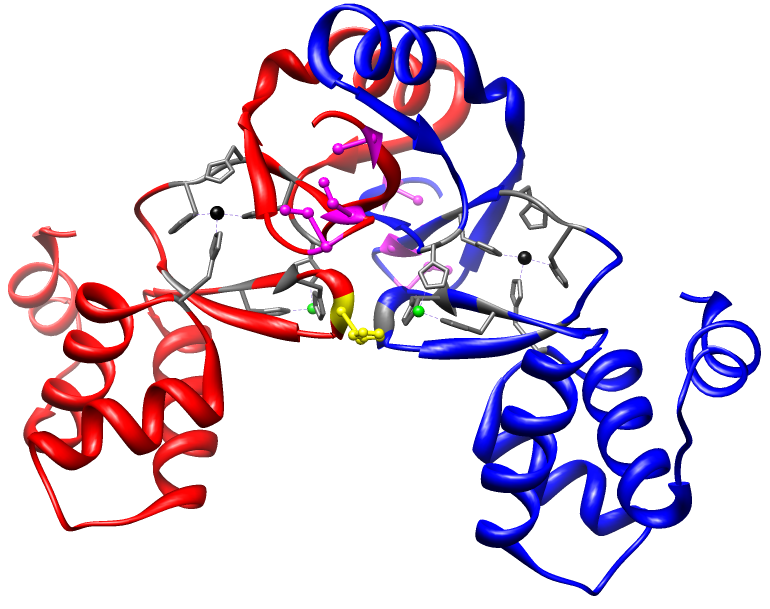
1. Characterize the metal and DNA binding properties of the Nickel Uptake Regulator (Nur)
Using the crystal structure of Nur as a guide (below), we are working to
biophysically characterize the metal binding and DNA binding chemistries of Nur. The goal of these experiments is to provide information about the allosteric coupling energies described above and understand the
mechanism by which the metal binding site communicates with the DNA binding
site.
2. Investigate putative metal regulatory proteins.
Using the common families of metalloregulators as the basis for database
searches, we have identified several candidate operons in the S. coelicolor A3(2) strain.
The genes will be cloned into appropriate expression plasmids and
transformed into E. coli.
The sensory metal for each of these candidate genes will be identified by
Electrophoretic Mobility Shift Assays in the presence of DNA and various metal
ions. The resulting pattern of band
shifts will indicate which, if any, metals are able to induce a dissociation or
association of the protein-DNA complex, which will directly guide future
experiments. Of particular interest
are proteins involved in sensing Zn2+ and Cu+.
Once identified, these proteins will be purified to homogeneity, and
thoroughly characterized biophysically.
![]()