CHEM 107 General Chemistry Lab I
Qualitative Analysis Scheme -Prelab
Before coming to lab, you should:
-
Read the assigned background reading
in Cooperative Chemistry Laboratory Manual and
in Chemistry and Chemical Reactivity (See laboratory
handout for page numbers.)
-
Answer the following questions on a
separate sheet of paper :(Turn in to your lab instructor at the
beginning of lab.)
-
(3 pts)
Define the following terms:
a) decant
b) supernatant
c) precipitate
-
(1 pt)
Consider the following chemical reaction:
AgNO3(aq)
+ NaCl(aq) ’
AgCl +
NaNO3
In this chemical reaction, a precipitate formed. What is the precipitate that formed?
- (1 pt) This is a qualitative experiment. What does this mean? What will you be looking for in this experiment?
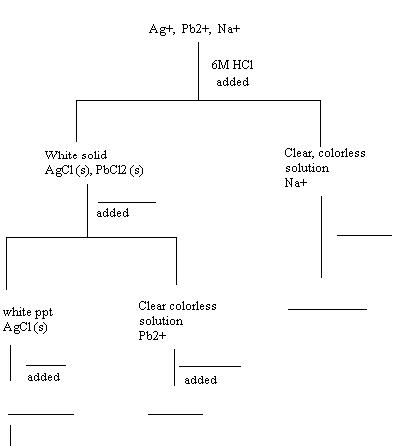
- (5 pts) The following problem is an example of a qualitative analysis experiment using precipitate reactions to remove ions sequentially from a mixture. This example will familiarize you with a flow chart, which is a graphical representation of the procedures and the results.
The mixture contains 3 ions, Ag+, Pb2+, and Na+. The three ions must be separated from this solution. This experiment will only be done on paper in order to familiarize you with the process of generating a flow chart.
STEP 1: Procedure
· Add 4 drops of 6M HCl to 10 drops of the test solution (the test solution is the solution containing all three ions, Ag+, Pb2+, Na+). Decant the supernate into a clean test tube and save for STEP 3 and save the ppt for STEP 2.
STEP 1: Flow Chart
· When HCl is added to the test solution, a precipitate (ppt) forms in the bottom of the test tube. What is this ppt? Use the Solubility Guidelines on page 60 of your lab book to determine what solid formed.
· Notice from the Solubility Guidelines that all chlorides are soluble except Ag+ and Pb2+ ions. Therefore, the following reactions took place when HCl was added to the test solution:
§ Ag+ + Cl- ® AgCl(s)
§ Pb2+ + Cl- ® PbCl2(s)
· The Na+ ion remains in solution. Now we have a test tube with a liquid and a solid. The liquid was separated from the solid.
· Next, this process must be represented in a flow chart before continuing with the experiment. In the flow chart, you want to record procedures, results, and observations. The flow chart for step 1 is done for you.

STEP 2: Procedure
· Add 15 drops of distilled water to the ppt obtained in STEP 1 and place the test tube in a hot water bath. Centrifuge and decant the supernate. Save the ppt for STEP 3. To the supernate, add 3 drops of 1M Na2CrO4. The formation of a yellow ppt, PbCrO4, confirms the presence of Pb2+.
STEP2: Flow Chart
· PbCl2 is soluble in hot water while AgCl is not. Therefore, the test tube now contains a liquid and a ppt. The ppt is _______________ and the supernate contains the ___________ ion.
· Next, this process must be represented in a flow chart before continuing with the experiment.
· Complete the flow chart to represent the procedures and results from step 2. Indicate in the flow chart, that hot water was added to the ppt from step 1. Next, indicate that the ppt contains the silver ion and that the supernate contains the Pb2+ ion. Next, indicate that 3 drops of 1 M Na2CrO4 was added to the supernate (which contains the lead ion). A yellow ppt (PbCrO4) formed confirming the presence of Pb2+.
STEP 3: Procedure
· Add 10 drops of 6M NH3 to the ppt from step 2. The ppt must be completely dissolved.
· Next, add 20 drops of 6M HNO3. The solution must be acidic. Stir the solution and test its acidity. Continue to add HNO3 dropwise until the solution is acidic. A white cloudiness confirms the presence of Ag+.
STEP 3: Flow Chart
· Complete the flow chart to represent the procedures and results from step 3.
STEP 4: Procedure
· To confirm the presence of Na+, perform a flame test. The sodium ion will impart a characteristic yellow color to the flame.
STEP 4: Flow Chart
· Complete the flow chart to represent the procedures and results from step 2.