Joule's Law and Heat Transfer Name: ________________________
A. Joule's equivalent of heat
Purpose: To measure the Joule's equivalent of heat by the electrical method.
Apparatus: PC and interface, temperature sensor, heating coil, power source (transformer), calorimeter: jacket and cup, electronic balance, cold-water (<20oC), ice, digital multi-meters (2), and banana-plug wires (5: 2-Red and 3-Black).
Theory: We will use electrical energy to heat a certain amount of cold-water. Electrical energy is measured in Joules and heat is measured in calories. In this activity we will look at a version of Joule's experiment, which gives a relationship between calorie and Joule.
To do this we need to measure the amount of electrical energy we supply and the amount of heat energy the water, calorimeter-cup, and heater gains. Then neglecting heat loss to the room, we could find the relationship between calorie and Joule.
Electrical energy, E is given by: E = I V t;
where I = current, V = voltage, and t = time.
Thermal energy or Heat gained, Q is given by: Q = MwCwΔT + McCcΔT +MhChΔT;
where Mw is the mass of water, Cw is the specific heat of water, Mc is the mass of the calorimeter cup, Cc is the specific heat of the calorimeter cup, Mh is the mass of the heater, Ch is the specific heat of the heater, and ΔT is the change in temperature.
Joule's equivalent of heat, J is given by; J = E/Q.
Procedure:
In order to measure the current and voltage, we need to set up the following circuit:
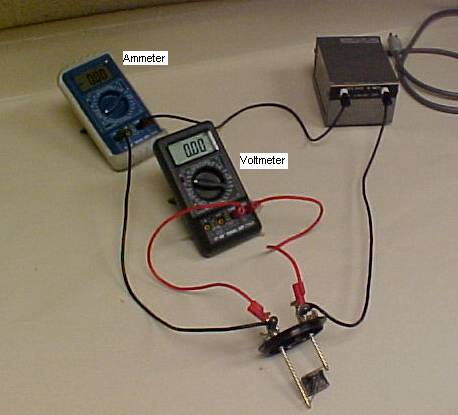
1. While the power cord of the transformer is unplugged, connect the
transformer, ammeter (DMM-1), and the heater in a series circuit.
2. Connect the voltmeter (DMM-2) across the heater.
3. Set DMM-1 to measure ac-current in the 10-A range and set the DMM-2 to measure ac-voltage in the 20-V range.
4. Call the instructor to check your circuit construction.
5. Find the mass of the calorimeter cup, and fill it with cold water (below 20 deg. C) about 4/5 full, and find the mass of the cup with water.
6. Place the calorimeter cup inside the jacket, immerse the heater coil in the water, and immerse the temperature sensor in the water as shown below.

7. Open DataStudio, select "Open Activity", select "Library", select "Physics Labs folder", and select "P16-Temperature and Heat". Click on the digits display and click start.
8. Plug in the power, and stir the water gently with the temperature sensor.
9. When the temperature reaches 20oC, the PC will start collecting the temperature data and the data collection will stop after 10 minutes.
10. It is important that you stir the water well during the experiment,
especially just before 20oC and just before the time reaches 10
minutes. Explain Why?
________________________________________________________________________
________________________________________________________________________
11. Record the current and voltage readings and complete the data table.
12. Unplug the transformer power cord and disconnect the circuit.
DATA:
Mass of calorimeter cup = Mc = __________ Mass of cup + water = __________
Mass of water = Mw =_______ Current = I = __________ Voltage = V = ___________
Change in temp. = ΔT = ______ Time = t = 600 s
Electrical energy supplied = E = I V t = ___________ Joule
Heat energy gained = Q = MwCwΔT + McCcΔT
+ MhChΔT = ___________ calorie
(Use Cw = 1 cal/(g.Co), Cc = 0.215 cal/(g.Co),
MhCh= 2.5 cal/Co)
Assuming no heat loss to the room; Q calories = E Joules.
Experimental value: 1 calorie = __________ Joule.
Theoretical value: 1 calorie = 4.186 Joule.
% Error = _____________________
13. Write a conclusion.
B. Heat Transfer
Purpose: To investigate what properties affect the heat transfer from a hot object.
Apparatus: Temperature sensors (2), PC w/interface, two cans: one painted black, hot plate, beaker, gloves, and water.
Background Information:
1. List the three methods of heat transfer and give an example for each.
|
Method of Heat transfer |
An example |
|
|
|
|
|
|
|
|
|
2. Describe the difference in the absorption of heat by radiation for a black painted object versus polished-metallic object. ________________________________________________________________________
________________________________________________________________________
3. Describe the difference in the emission of heat by radiation for a black painted object versus polished-metallic object. ________________________________________________________________________
________________________________________________________________________
Procedure:
1. Connect one temperature sensor to analog channel A and the other to analog channel B.
2. Place the A probe in the unpainted can and B probe in the painted can, and place the cans on a paper towel.
3. Open "Data Studio", select "Open Activity", select "Library", select "Physics Labs", and select P46, Heat Transfer.
4. Click "temperature versus time graph display".
5. Carefully pour equal amount of hot water into the two cans, and click "Start".
6. Data collection will stop automatically after 15 minutes.
7. Maximize the graph display.
8. Print a hard-copy the temperature versus time graph.
Questions:
1. Which can cooled down faster?
2. When the cans are cooling, which processes transfer heat?
3. When the cans are cooling, which process do you think is dominant? Why?
4. When a can is cooling, does it cool faster at the beginning or towards the end? Explain Why? (Include an appropriate data from your measurement in your explanation)