b.
1. n-octane and 2,2,4-trimethyl pentane both have the same molecular formula C8H18.
a. Draw the molecular structure of each of these compounds showing all bonds and atoms.
b. Look up the boiling points for each of these and explain why they are different; to support your answer, include a diagram showing the attractions that have to be broken for these individual substances to boil.
2. Draw the Lewis and molecular structures for each of the following show all bonds, nonbonding pairs, atoms, and nonzero formal charges:
a. N(CH3)4+
b. CH2CHCH3
c. CH2OHCHOHCH2OH
d. CCl3COO-
e. CF2HCHO
f. NH2NH2
g. CH3CONH2
h. C2H5OCH3
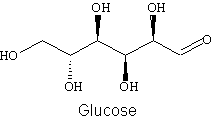
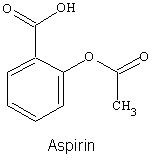
3. Draw all valence lone electron pairs and then use arrows to indicate on the following molecules every point at which hydrogen bonds can be donated (HBD) or hydrogen bonds can be accepted (HBA).
a.

b.

4. Calculate the fraction of gaseous argon atoms that has a kinetic energy greater than 10 kJ/mole at a temperature of 10,000 K.