Publications Presentations Funding People
Research
Interests
Dr.
Lammi and her students use molecular spectroscopy – in bulk solution and at the
level of single particles – to investigate research questions of interest.
Current studies focus on (A) folding, oligomerization, and aggregation
inhibition in Alzheimer’s amyloid-b peptide (Ab) and (B)
fluorescence spectroscopy of ZnO nanoparticles.
A. Folding,
Oligomerization, and Aggregation Inhibition in Alzheimer’s Amyloid-b Peptide
Amyloid-b (Ab) is a peptide
of 39-43 amino acids that is created by the enzymatic cleavage of the amyloid
precursor protein (APP). It readily self-assembles into a diverse array of
oligomers and aggregates which are thought to be causative agents in
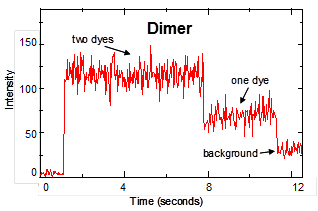
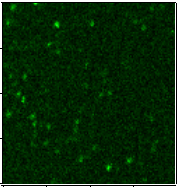
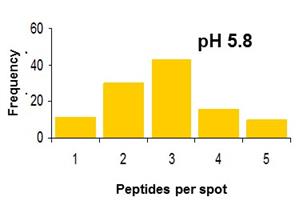
Alzheimer’s disease. Our group has utilized single-particle fluorescence
spectroscopy to monitor oligomerization, one peptide species at a time, and to
probe structures and conformational change in individual Ab dimers. We
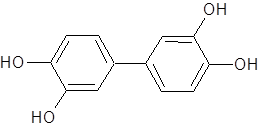
are currently collaborating with Dr. Hanna’s group in the design, synthesis,
and evaluation of small-molecule inhibitors of Ab aggregation.
B. Fluorescence
Spectroscopy of ZnO Nanoparticles
Zinc
oxide is a II-VI semiconductor with a direct band gap of 3.37 eV (375 nm). Its
high electron mobility and strong luminescence make it favorable for use in
light-emitting diodes (LEDs), among other applications. Nanoparticles of ZnO
are among the most commonly produced nanomaterials, used extensively in
sunscreen (where they absorb UV light); ZnO nanoparticles (NPs) are also being
widely studied for their antimicrobial properties. Dr. Gelabert’s lab is
synthesizing ZnO NPs by hydrothermal methods; we are collaborating on their
spectroscopic characterization, via steady-state and time-resolved bulk
fluorescence and single-particle methods.