Organic Methodology
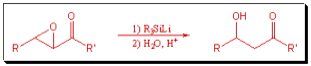 The development of new reactions is extremely important, particularly in drug discovery and the manufacture of pharmaceuticals. We are specifically interested in the application of organosilicon reagents in organic synthesis. Our current research focuses on the use of organosilylmetallic reagents and on rearrangements involving migration of silicon directed toward the development of new synthetic organic methods. We have already developed a method for preparing β-hydroxyketones (aldols) by the selective α-reduction of α,β-epoxyketones using silyllithium reagents
The development of new reactions is extremely important, particularly in drug discovery and the manufacture of pharmaceuticals. We are specifically interested in the application of organosilicon reagents in organic synthesis. Our current research focuses on the use of organosilylmetallic reagents and on rearrangements involving migration of silicon directed toward the development of new synthetic organic methods. We have already developed a method for preparing β-hydroxyketones (aldols) by the selective α-reduction of α,β-epoxyketones using silyllithium reagents
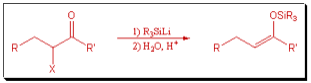 We have also developed methods that utilize silyllithium reagents for the regio- and stereoselective preparation of simple and functionalized silyl enol ethers. These are important and versatile intermediates, particularly for use in carbon-carbon bond forming reactions.
We have also developed methods that utilize silyllithium reagents for the regio- and stereoselective preparation of simple and functionalized silyl enol ethers. These are important and versatile intermediates, particularly for use in carbon-carbon bond forming reactions.
Chemical Education
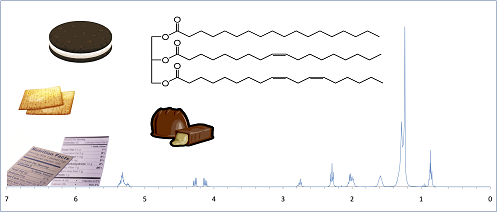 We are interested in developing new experiments which can be incorporated into undergraduate laboratory courses. In particular, we try to create experiences that are relatable to students by incorporating familiar materials into the experiments. We recently created an experiment for our organic laboratory course (CHEM 304) in which students extract the fat content from common 'convenience' foods and analyze it using 1H-NMR.
We are interested in developing new experiments which can be incorporated into undergraduate laboratory courses. In particular, we try to create experiences that are relatable to students by incorporating familiar materials into the experiments. We recently created an experiment for our organic laboratory course (CHEM 304) in which students extract the fat content from common 'convenience' foods and analyze it using 1H-NMR.
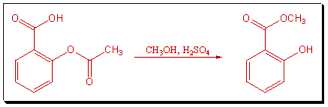 We also created an experiment for our organic laboratory course (CHEM 304) in which commercial aspirin tablets are converted to Oil of Wintergreen.
We also created an experiment for our organic laboratory course (CHEM 304) in which commercial aspirin tablets are converted to Oil of Wintergreen.
We have also developed a short synthesis of nicotine. The seven-step route utilizes a balance of “sophomore” organic chemistry such as Grignard addition and hydrogenation and more advanced reactions like ring closing metathesis (RCM). The synthesis was developed for use in our advanced synthesis laboratory course (CHEM 570).